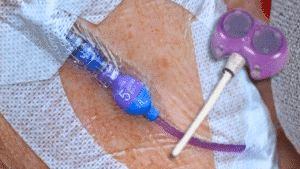
The Bard PowerPort lawsuit involves allegations that a widely used implantable port catheter device was defectively designed and manufactured, leading to serious and sometimes life-threatening complications. Patients across the United States have reported fractures, migration, vascular damage, blood clots, embolisms, infections, and strokes linked to Bard PowerPort devices.
Salenger, Sack, Kimmel & Bavaro, LLP is currently accepting new Bard PowerPort claims on behalf of individuals who were injured after implantation of a Bard port or catheter device.
This page explains the litigation, eligibility criteria, known complications, MDL updates, bellwether trial dates, and realistic—but preliminary—settlement expectations.
The Bard PowerPort (sometimes referred to as a port-a-cath or implantable venous access port) is a medical device surgically implanted beneath the skin to provide long-term intravenous access for medications, fluids, chemotherapy, or blood draws.
Manufactured by Bard, the PowerPort was marketed as a durable, high-pressure injectable port. However, lawsuits allege that design and material defects made the device prone to fracture, degradation, and migration, exposing patients to significant medical risks.
Patients injured by defective Bard PowerPort devices are bringing product liability lawsuits alleging that:
These cases have now been centralized in federal court as part of a multidistrict litigation (MDL).
January 6, 2026 – Over 2,500 Cases Pending
As of January 2026:
The litigation is pending before Judge David Campbell in the U.S. District Court for the District of Arizona and continues to mature with discovery disputes resolved and bellwether trials scheduled.
The Bard PowerPort mass tort (MDL 3081) centers on alleged design defects in the catheter tubing and materials, which plaintiffs argue lead to fracture, migration, thrombosis, infection, and vascular injury.
A key issue in the litigation is Bard’s reliance on the FDA 510(k) clearance process, which allowed the PowerPort to enter the market in 1999 without clinical testing for long-term safety or durability. Plaintiffs allege that Bard prioritized market speed over patient safety.
Recent filings include disputes over sales representative disclosures, trial scheduling, and compliance with discovery obligations. Bellwether trials are expected to drive future settlement discussions.
The following bellwether cases are currently scheduled:
These trials will test key liability and causation theories and may shape future settlement negotiations.
If you or a loved one has experienced health complications due to the Bard powerport catheter, don’t wait to seek legal help.
Contact Salenger, Sack, Kimmel & Bavaro today for a FREE consultation.
Call (800) 675-8556 or contact us online to get started.
You may qualify for a Bard PowerPort lawsuit if:
Medical records confirming implantation and injury are typically required.
Fracture is the most commonly reported PowerPort failure. Plaintiffs allege that the catheter tubing becomes brittle over time, allowing pieces to crack or break off.
Once fractured, device fragments can migrate through the bloodstream, leading to:
Fracture-plus-migration cases are generally considered the strongest and highest-value claims.
Migration occurs when the catheter or fractured components move from their intended location. This can happen independently or in combination with a fracture.
Migrated fragments may lodge in the heart, lungs, or major blood vessels, creating life-threatening risks and often requiring invasive intervention.
Although less intuitive, infections are another major complication associated with PowerPort devices. Plaintiffs allege that material degradation creates microscopic cracks where bacteria can grow.
Reported complications include:
Infection cases are generally harder to prove but remain viable under the right medical circumstances.
Any discussion of settlement amounts at this stage is inherently speculative and should be approached with caution. That said, based on comparable mass tort litigation and injury severity, attorneys estimate the following potential settlement tiers:
Fracture + Migration with Vascular Injury
Estimated range: $175,000 – $350,000
Thrombosis or Pulmonary Embolism
Estimated range: $100,000 – $250,000
Infection-Only Cases
Estimated range: $30,000 – $100,000
Some severe cases may exceed these ranges, while others may resolve for less depending on causation evidence and medical history.
Cases that proceed to trial may present substantially higher risk—and reward. Successful verdicts could include:
Plaintiffs’ attorneys believe successful verdicts could exceed $10 million in certain cases. However, trial outcomes are uncertain, and no result is guaranteed.
Statutes of limitation apply to Bard PowerPort claims and vary by state. Waiting too long could bar your claim entirely.
Early participation also ensures:
The Bard PowerPort lawsuit alleges that certain implantable port catheter devices were defectively designed and manufactured, making them prone to fracture, migration, and material degradation. These failures have been linked to serious injuries such as vascular damage, blood clots, embolism, infection, and stroke.
As of January 2026, more than 2,500 active cases are pending in the federal MDL, with additional cases proceeding in state courts nationwide.
Bellwether trials are scheduled to begin in April 2026 and continue through early 2027. These trials are intended to test evidence and may influence future settlement negotiations.
Settlement values vary widely based on the type of device failure and resulting injury. While outcomes are not guaranteed, estimates range from tens of thousands of dollars for infection-only claims to hundreds of thousands of dollars for fracture and migration cases involving vascular injury. Severe cases may exceed these ranges.
Not at all! Deadlines vary by state and depend on when the injury was discovered. Statutes of limitation may apply, so it is important to speak with a lawyer as soon as possible to avoid losing your right to file a claim.
No, The Bard PowerPort cases are handled on a contingency fee basis, meaning there are no upfront legal fees. Attorney fees are only paid if compensation is recovered.
The first step is a free case evaluation with a product liability attorney. You will typically be asked about your medical history, device implantation, injuries, and available records to determine eligibility.
If you or a loved one suffered complications from a Bard PowerPort device, you may have legal options.
Salenger, Sack, Kimmel & Bavaro, LLP offers free, confidential case evaluations and works on a contingency fee basis—you pay nothing unless compensation is recovered.
👉 Contact us today to see if you qualify for a Bard PowerPort lawsuit.